OuluHealth Investing to Overcome the Early Stage Regulatory Challenges of Their Members
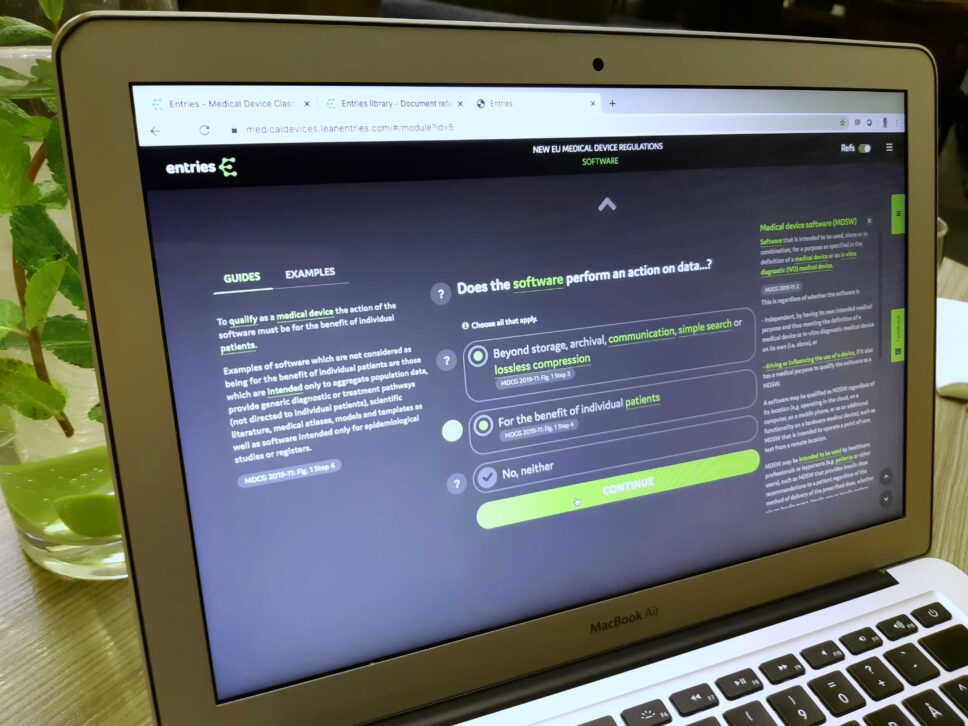
It is well known that regulatory compliance is one of the major challenges for health tech companies, especially for the ones starting up. This is a topic OuluHealth and other health tech stakeholders routinely discuss. Now the topic is even more important than before as the new MDR will come into force bringing new requirements for the companies, especially in the area of digital health. “Often the medical device regulations are studied rather late in the innovation process.” points out Minna Komu, the Network Director of OuluHealth. “It may not even be clear for the innovator, whether the product or software is regulated as a medical device, which makes a world of difference in their business.”
It takes a substantial amount of time and focus to become sufficiently informed on regulations and standards. And even more to turn both into a business advantage. Acknowledging this challenge, and finding means to overcome it, is in the interests of OuluHealth. “To build success stories, we need to make the local companies, university innovators, investors and related stakeholders more educated on topics, that may be overwhelming for the non-expert, but crucial for making business.” says Komu.
Lean Entries Ltd was found and hired by OuluHealth due to their intuitive digital solution (Entries) to solve the early stage regulatory challenge and their mission to empower health tech startups. In their trainings, they make the complex regulatory topic resonate with competitive gains and modern business practice. Their one-on-one regulatory clinics have put our community startups and innovators on an informed track towards compliance. As a result, the investment made by OuluHealth has already paid back after one month into the program that spans a full year.
“OuluHealth is a particularly well organised ecosystem, keeping all stakeholders well in their loop. This helps greatly in sharing knowledge efficiently to the ones in need.” describes Heikki Pitkänen, the CEO and Founder of Lean Entries Ltd. “Hear me on Medical Device Qualification and Classification according to the MDR and IVDR on the Easy Medical Device podcast!”
